
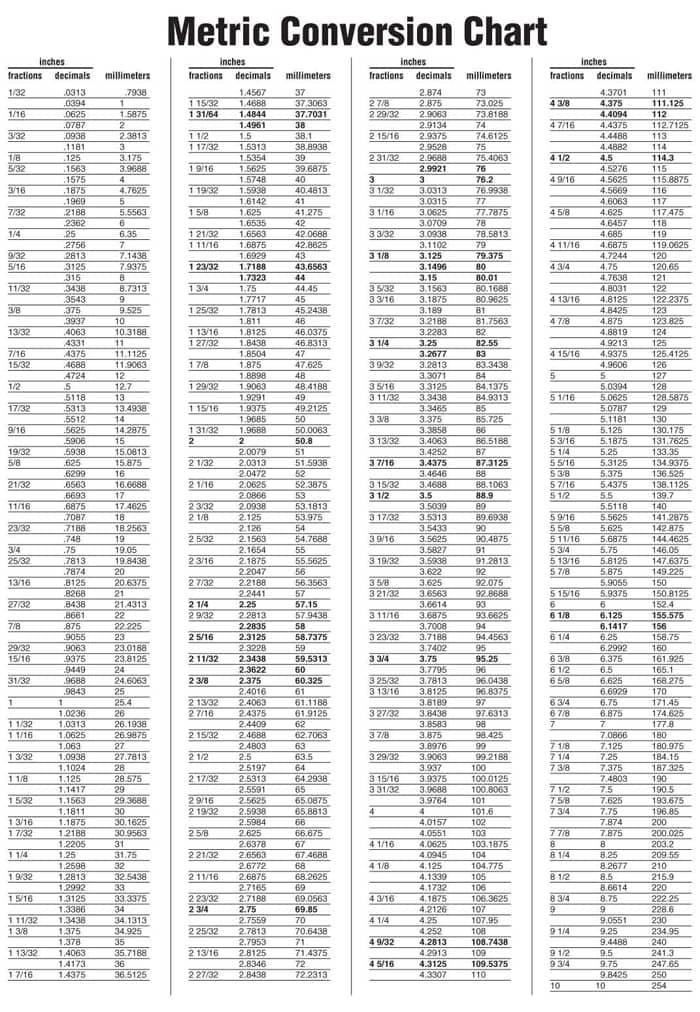
Question 5: A chloride ion has a radius of 181 pm. Convert this to a radius in picometres.įrom the table above we see that 1 m = 10 12 pm Question 4: A sodium ion has a radius of 1.02 × 10 -10 m. Multiply both sides of the equation by 220 Convert this to a radius in metres.įrom the table above we see that 1 pm = 10 -12 m Question 3: An atom of iodine has a radius of 220 pm. Multiply both sides of the equation by 500 Convert this to a wavelength in metres.įrom the table above we see that 1 nm = 10 -9 m Question 2: A beam of yellow light has a wavelength of 500 nm.

Question 1: Convert a bond length of 1.4 × 10 -10 m to nanometres.įrom the table above we see that 1 m = 10 9 nm Play the game now! Worked Examples of Length Conversions Using SI and Metric Units Number of picometres in a metre = 1 pm ÷ 10 -12 = 10 12 pm/mįor chemistry students, the most important conversions are probably between metres (m), nanometres (nm) and picometres (pm): Number of nanometres in a metre = 1 nm ÷ 10 -9 = 10 9 nm/m Number of micrometres in a metre = 1 μm ÷ 10 -6 = 10 6 μm/m Number of millimetres in a metre = 1 mm ÷ 10 -3 = 10 3 mm/m Number of centimetres in a metre = 1 cm ÷ 10 -2 = 10 2 cm/m Number of kilometres in a metre = 1 km ÷ 10 3 = 10 -3 km/m We can use this determine how many of each unit are in a metre: 10 -6 m is called 1 micrometre (μm, previously known as a micron).
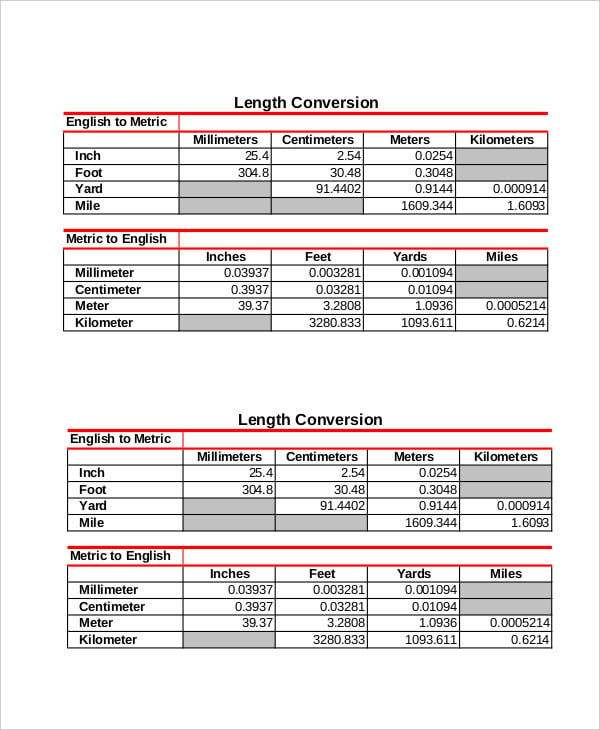
If an atom with has a radius of 1.54 Å we can convert this radius to metres as shown below: The diameter of a nanoparticle, like a buckyball, which is generally between 10 -9 and 10 -7 metresĪll of the lengths listed above are much, much, smaller than a metre.īecause the size of an atom and the distance between atoms in a molecule is of the order of 10 -10 metres, a convenient unit of measurement is the Ångstrom (Å) (3) defined as 10 -10 m.The wavelength of X-rays which are of the order of 10 -10 metres.The wavelength of visible light (distance between adjacent wave peaks) which is of the order of 10 -7 metres.The distance between atoms in a molecule which is of the order of 10 -10 metres.The size of an atom which is of the order of 10 -10 metres.My ruler is 30 centimetres long and each centimetre is divided into 10 millimetres.īut all the distances above are large enough to see.Ĭhemists are interested in atoms and the distances between them which are much too small to be seen using only your eyes.įor example, some of the lengths, or distances, chemists are interested in are: We measure the height of a person in metres or centimetres. We measure the distance between one town and another in kilometres. You are probably already very familiar with some units for measuring distance.
#METRIC DISTANCE CONVERSION FREE#
No ads = no money for us = no free stuff for you! Units of Length and Unit Conversions


 0 kommentar(er)
0 kommentar(er)
